One of the first things I did when I arrived in Dunedin for the first time in 2012 was to go to the Royal Albatross Centre. This place is the only mainland colony in the word where you can observe the Northern Royal Albatross in their natural habitat. Particularly impressive when they glide, the albatrosses are also interesting for their snot.
Yes, you read that correctly. The albatross’ snot could help us to imagine and develop a new desalination technique.
It is well known that drinking seawater is unhealthy. The amount of salt is so high that it will dehydrate your body. As a result you will be dehydrated pretty quickly, and if you don’t get fresh water soon enough you will get ill and even die.
Ok, but what about seabirds? Their main water resource is from seawater, and they look fine. They are fine. How so?
Researchers were curious to know how seabirds could drink seawater. They analysed their urine and only found small amount of salt. They thought maybe they are not drinking seawater! To check the hypothesis they made albatrosses drink seawater and observe that they were totally fine with it. They finally discover a HUGE amount of salt in the birds’ snots, or should I say, in the fluid coming out of their nostrils.
How do they do that? Snots glands! Nah, I’m kidding. A pair of nasal glands produce the salt solution coming out of their nostrils. In other species drinking seawater like sea turtles or iguanas, the same function is accomplished by the salt glands or supraorbital glands in penguins.
If you have a look at the picture below you can see that there is transportation of the salt (NaCl) through secretory cells from the blood to secretory tubules, which will excrete the super salty fluid by the nostril.
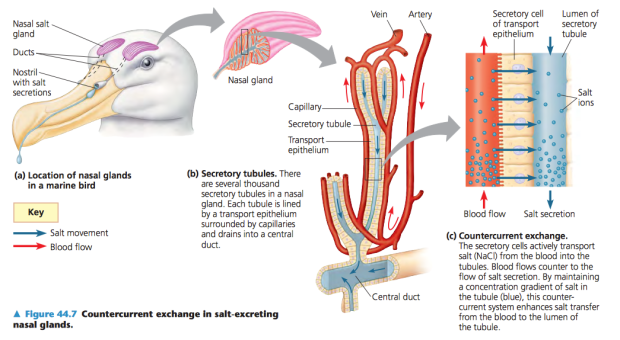 Credit: Pearson Education, Inc. publishing as Benjamin Cummings
Credit: Pearson Education, Inc. publishing as Benjamin Cummings
Thanks to the nasal glands, the albatross are able to drink seawater and get water out of it, where we would lose more water in the process of trying to eliminate the excess of salt!
Now, the challenge is to work out a process that could do the same with a low environmental impact and low manufacturing cost (in a perfect world I guess). And, once more, another good solution would be to consume less water ;).
Through this post, I wanted to show you that besides all the technologies already based on Nature; there is still much more to learn!
Nature is a bottomless source of inspiration. Take the time to go outside and observe the world. Get inspired and stay amazed by what Nature has to offer. None of what is around is there by mistake or by chance. Every piece of Nature is the product of millions years of Evolution. Appreciate it.


 Eyes compilation (from left to right): Dragonfly, Longhorned beetle, Robber fly and Horse fly.
Eyes compilation (from left to right): Dragonfly, Longhorned beetle, Robber fly and Horse fly.








 Credit: Michael Phillips
Credit: Michael Phillips
 Larval stage of the fish parasite Pomphorhynchus tereticollis. (A) Pomphorhynchus tereticollis (B) Detail of proboscis. Scale bar = 500 µm.
Larval stage of the fish parasite Pomphorhynchus tereticollis. (A) Pomphorhynchus tereticollis (B) Detail of proboscis. Scale bar = 500 µm.





 Credit: Erwin Poliakoff, Doug Anderson, Keefer Milton / Flickr.
Credit: Erwin Poliakoff, Doug Anderson, Keefer Milton / Flickr.




 Aeroplane prototype
Aeroplane prototype





 Credit:
Credit: 
